Konstantinos Rigos / National Hellenic Research Foundation, Athens, Greece
Pavlos Saridis / National Hellenic Research Foundation, Athens, Greece
Georgios Filis / National Hellenic Research Foundation, Athens, Greece
Dimitra Zarafeta / National Hellenic Research Foundation, Athens, Greece
Georgios Skretas / Biomedical Sciences Research Centre “Alexander, Fleming”, Vari, Greece
Topic: Novel Enzymes
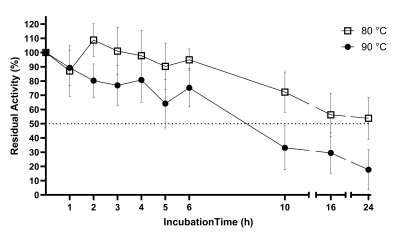
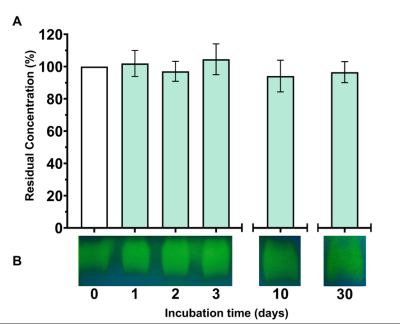
Rising CO2 emissions have exacerbated the greenhouse eect, prompting the EU's pledge for net-zero emissions by 2050; rendering sustainable CO2 Capture and Storage Technologies more urgent than ever. Benchmark technologies rely on amines which present toxicity and high energy cost. An eco-friendly sustainable alternative involves the use of carbonic anhydrases (CAs), enzymes, ubiquitous in nature that catalyze CO2 hydration reaction. Conventional enzymes cannot withstand the harsh conditions (high T and pH etc.) present in industrial CO2 capture units. Thus, the discovery of ultra-stable CAs is necessitated. Herein an ultra-stable CA was discovered through large-scale bioinformatic analysis of metagenomic datasets from the SRA and JGI databases. CA-KR1was produced recombinantly in E.coli and biochemically characterized. The novel CA-KR1 is one of the most thermostable and alkali-stable CAs known to date, making it an exceptional candidate biocatalyst for industrial CO2 sequestration.