Laccases are oxidoreductases belonging to the multinuclear copper-containing oxidases. The overall outcome of their catalytic cycle is the reduction of one molecule of oxygen to two molecules of water and the concomitant oxidation of four substrate molecules to give four radicals.1
Typical substrates of laccases are phenols and aliphatic or aromatic amines, the reaction products being mixtures of dimers or oligomers derived by the coupling of the reactive radical intermediates. For instance, this biotransformation has been exploited to isolate new dimeric derivatives of natural phenolic derivatives (resveratrol and its analogues, chalcones, …).2
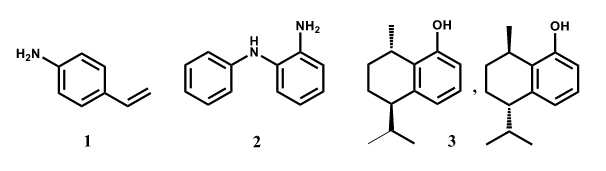
As far as aromatic amines concerns, we have recently reported the results obtained in the oxidation of vinyl amines (i.e., 1)3 and we are currently investigating the oxidation of ortho-substituted diamines, i.e. the previously described compound 2.4
In other studies, years ago we described the use of laccase-catalyzed reactions for the selective C-C coupling of highly substituted phenols, like β-estradiol and totarol.5 Recently updates of this biocatalyzed reactions have been obtained with the enantiomers of trans-8-hydroxycalamenene (3)
References
[1] a) S. Riva, Trends Biotechnol. 24: 219 (2006); b) I. Bassanini, E.E. Ferrandi, S. Riva, D. Monti, Catalysts, 11: 26 doi.org/10.3390/catal11010026 (2021)
[2] a) S. Nicotra, M. Cramarossa, A. Mucci, U. Pagnoni, S. Riva, L. Forti, Tetrahedron 60: 595 (2004); b) S. Nicotra, A. Intra, G. Ottolina, S. Riva, B. Danieli, Tetrahedron-Asymmetry 15: 2927 (2004); c) C. Ponzoni, E. Beneventi, M. Cramarossa, S. Raimondi, G. Trevisi, U. Pagnoni, S. Riva, L. Forti, Adv. Synth. Catal. 349: 1497 (2007); d) S. Ncanana, L. Baratto, L. Roncaglia, S. Riva, S. Burton, Adv. Synth. Catal. 349 : 1507 (2007); e) P. Gavezzotti, C. Navarra, S. Caufin, B. Danieli, P. Magrone, D. Monti, S. Riva, Adv. Synth. Catal., 353: 2421 (2011); f) C. Navarra, P. Gavezzotti, D. Monti, W. Panzeri, S. Riva J. Mol.Catal. B-Enzymatic, 84 : 115 (2012); g) P. Gavezzotti, F. Bertacchi, G. Fronza, V. Křen, D. Monti, S. Riva, Adv. Synth. Catal., 357: 1831 (2015); h) I. Bassanini, I. D’Annessa, M. Costa, D. Monti, G. Colombo, S. Riva Org. Biomol. Chem., 16: 3741 (2018); i) S. Grosso, F. Radaelli, G. Fronza, D. Passarella, D. Monti, S. Riva, Adv. Synth. Catal., 361: 2696 (2019).
[3] I. Bassanini, S. Grosso, C. Tognoli, G. Fronza, S. Riva, Int. J. Mol. Sci., 24: 3524 (2023).
[4] A. Catarina Sousa, M. Conceição Oliveira, Lígia O. Martins, M. Paula Robalo Adv. Synth. Catal. 360, 575 (2018).
[5] a) S. Nicotra, A. Intra, G.Ottolina, S. Riva, B. Danieli, Tetrahedron Asymmetry, 15: 2927 (2004). b) S. Ncanana, L. Baratto, L. Roncaglia, S. Riva, S. G Burton Adv. Synth. Catal. 349, 507 (2007)
 Dott. Sergio Riva has been working as a scientist of the Italian National Council of Research (C.N.R.) since 1984, and is presently Director of Research (Direttore di Ricerca) at the Institute of Chemical Sciences and Technologies (SCITEC).
Dott. Sergio Riva has been working as a scientist of the Italian National Council of Research (C.N.R.) since 1984, and is presently Director of Research (Direttore di Ricerca) at the Institute of Chemical Sciences and Technologies (SCITEC).
He has been “Professore a Contratto” (Adjunt Professor) at the University of Modena and Reggio Emilia from 2000 to the present year.
He took his Laurea in Chemistry at Milano University in 1983 and his Diploma di Specialità in Organic Synthesis at Milano Politecnico in 1989. He spent one year (1987) at M.I.T. (Cambridge, USA) working with Prof. A. Klibanov on the forefront research line (at that time) of biocatalysis in organic solvents.
In 1993 he was awarded the Giacomo Ciamician medal and in 2023 the Adolfo Quilico medal by the Organic Chemical Division of the Italian Chemical Society for his research in the area of biocatalysis.
His scientific activity is documented by more than 220 publications reporting on the isolation and characterization of different groups of enzymes and on the synthetic exploitation of these biocatalysts.