GtHNL is a hydroxynitrile lyase that was successfully utilized for the synthesis of cyanohydrins (Scheme 1B) and nitro aldol products, in batch and in flow [1,2,3]. GtHNL has moderate similarity (35%) with Tm1459 and the same catalytic metal ion, Mn(II) (Scheme 1A). Enzymes display high selectivity for one type of reaction. Therefore, lyases are not expected to catalyze oxidative C=C bond cleavage reactions as Tm1459 does (Scheme 1C) [4]. However, examining the structure of GtHNL there is no obvious reason why it should not catalyze the oxidative C=C bond cleavage, as described for Tm1459. Equally a hydroxynitrile lyase activity for Tm1459 cannot be ruled out.
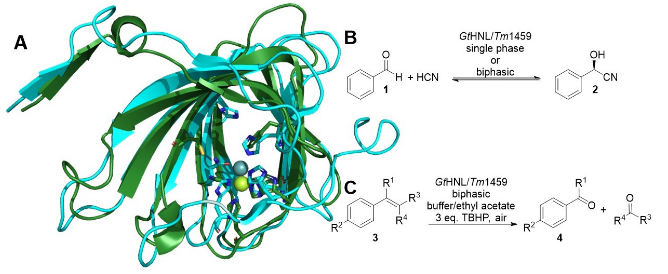
Scheme 1: A) Superimposition of GtHNL (PDB code: 4BIF; blue) with Mn(II) in grey coordinated by 5 amino acids and Tm1459 (PDB code: 1VJ2; green) with Mn(II) coordinated by only 4. B) Cyanohydrin formation. C) Oxidative C=C bond cleavage. GtHNL was employed under conditions earlier successful for Tm1459 [4]. tert-butyl hydroperoxide (TBHP) was used as oxidizing reagent and GtHNL satisfactorily catalyzed the oxidative cleavage of a number of styrene derivatives. Enzyme and reaction engineering led to improved selectivities and yields. On the other hand Tm1459 displayed only modest hydroxynitrile lyase activity without selectivity. These results question our concepts of enzyme selectivity and at the same time open new routes to novel enzyme activities [5].
____
[1] I. Hajnal, et al, FEBS J., 2013, 280, 5815–5828.
[2] J. Coloma, et al, Catal. Sci. Technol., 2020, 10, 3613–3621.
[3] J. Coloma, et al, Catalysts 2022, 12, 161.
[4] I. Hajnal, et al, Adv. Synth. Catal. 2015, 357, 3309 –3316.
[5] J. Coloma, et al, ACS Catal. 2023, 13, 11182−11194.
 Ulf Hanefeld received his PhD from the Georg-August-Universität zu Göttingen, having performed the research both with Prof. H. Laatsch (Göttingen) and Prof. H. G. Floss (Seattle). After postdoctoral years with Prof. C. W. Rees (Imperial College London), Prof. J. Staunton (Cambridge) and Prof. J. J. Heijnen and Dr. A. J. J. Straathof (TU Delft), he received a fellowship from the Royal Netherlands Academy of Arts and Sciences (KNAW). He rose through the ranks at the Technische Universiteit Delft and his research in Delft focuses on enzymes, their immobilisation and application in organic synthesis. In particular the environmentally benign synthesis Carbon-Carbon bonds are central to his research.
Ulf Hanefeld received his PhD from the Georg-August-Universität zu Göttingen, having performed the research both with Prof. H. Laatsch (Göttingen) and Prof. H. G. Floss (Seattle). After postdoctoral years with Prof. C. W. Rees (Imperial College London), Prof. J. Staunton (Cambridge) and Prof. J. J. Heijnen and Dr. A. J. J. Straathof (TU Delft), he received a fellowship from the Royal Netherlands Academy of Arts and Sciences (KNAW). He rose through the ranks at the Technische Universiteit Delft and his research in Delft focuses on enzymes, their immobilisation and application in organic synthesis. In particular the environmentally benign synthesis Carbon-Carbon bonds are central to his research.