Biocatalytic Synthesis of Pseudouridine: Optimizing Cascade Reactions from Milligram to Gram Scale
Abstract
C-Analogues of canonical N-nucleosides are crucial in medicinal chemistry. Synthetic C-nucleosides, like remdesivir, are well known for their antiviral properties, but their use also extends to serving as building blocks for synthetic RNA. The substitution of uridine with its C-nucleoside isomer pseudouridine (Ψ) is key to mRNA-based vaccine synthesis, as it makes the RNA less immunogenic, enhancing in vivo translation.1 While biocatalytic methods are well-established for N-nucleoside synthesis, they are lacking for C-nucleosides. The discovery of pseudouridine monophosphate C-glycosidase (YeiN) enables a biocatalytic synthesis route by catalyzing the selective 5-β-C-ribosylation of uracil from pentose 5-phosphates.2
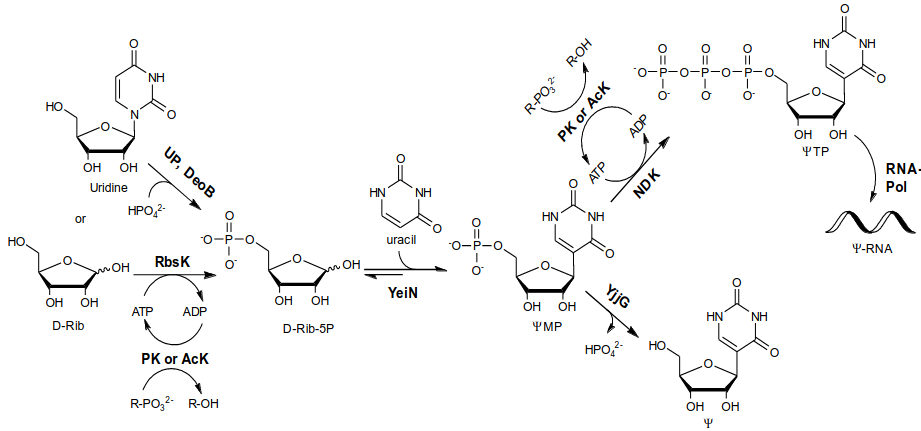
Figure 1. Phosphorylation-glycosylation cascades for one-pot synthesis of ΨMP and Ψ-5’-triphosphate (ΨTP) from unphosphorylated D-Rib. Dephosphorylation of ΨMP yields Ψ. UP uridine phosphorylase, DeoB phosphopentomutase, RbsK D-Rib-5-kinase, PK pyruvate kinase, AcK acetate kinase, YjjG nucleotide phosphatase, NDK nucleoside diphosphate kinase, RNA-Pol RNA-polymerase.
YeiN was integrated into a phosphorylation-C-ribosylation cascade, enabling efficient one-pot synthesis of C-nucleoside phosphates (yield: 20–95%) from unprotected sugars and nucleobases.2-3 By optimizing reaction conditions and switching the phosphate donor from phosphoenol pyruvate to acetyl phosphate, we achieved a ΨMP titer of up to 200 g/L. The phosphorylation-C-ribosylation cascade showed an impressive productivity of 38 g/L/h.3 Biocatalytic phosphorylation of ΨMP to ΨTP enabled its use in in vitro transcription to produce fully pseudouridinated mRNA.2 Furthermore, we developed a selective, atom-economic uridine-to-Ψ isomerization cascade.4 This one-pot process operates in four enzyme-catalyzed steps, via D-ribose-1-phosphate, D-ribose-5-phosphate, and ΨMP, to produce Ψ. The optimized isomerization achieved a productivity of 30 g/L, yielding a supersaturated Ψ solution (~250 g/L) from which pure Ψ crystallized, with a 90% recovery upon filtration.4 Collectively, these findings advance biocatalytic methodologies for C-nucleotide synthesis.
About the Speaker(s)
 Martin Pfeiffer is a postdoctoral researcher at the Institute of Biotechnology and Bioprocess Engineering, University of Technology Graz. He earned his PhD in Biotechnology from the same institution in 2020 with a thesis titled Carbohydrate-Active Enzymes: From Reaction Mechanism to Biocatalyst Design. He later worked as a postdoctoral fellow at the Austrian Centre of Industrial Biotechnology (ACIB) for one year before returning to the Institute of Biotechnology and Bioprocess Engineering in 2022. His research interests include the development of biocatalytic cascades, the molecular mechanisms of carbohydrate-active enzymes, and the discovery of novel enzymatic pathways.
Martin Pfeiffer is a postdoctoral researcher at the Institute of Biotechnology and Bioprocess Engineering, University of Technology Graz. He earned his PhD in Biotechnology from the same institution in 2020 with a thesis titled Carbohydrate-Active Enzymes: From Reaction Mechanism to Biocatalyst Design. He later worked as a postdoctoral fellow at the Austrian Centre of Industrial Biotechnology (ACIB) for one year before returning to the Institute of Biotechnology and Bioprocess Engineering in 2022. His research interests include the development of biocatalytic cascades, the molecular mechanisms of carbohydrate-active enzymes, and the discovery of novel enzymatic pathways.
