Improvement of α-amino ester hydrolase (AEH) via rational and computational design
Abstract
Current batch enzymatic processes to important semi-synthetic beta-lactam antibiotics, such as amoxicillin and cephalexin, suffer from yield and selectivity limitations, owing to primary and secondary hydrolysis side reactions (see Figure). Through continuous flow and reactive crystallization of the beta-lactam product, we sought to suppress primary hydrolysis and prevent secondary hydrolysis. Indeed, we found higher yields than in homogenous batch reactions. Amino ester hydrolase (AEH) is a potential alternative to the standard Pen G acylase (PGA) for the synthesis of some semisynthetic beta-lactam antibiotics, such as cephalexin. While AEH is more active and selective than PGA towards the synthesis of targets with (R)-phenylglycyl side chains than PGA, its substrate specificity is limited, its biophysical behavior is complex, and it is unstable at temperatures > 25oC. The presentation will describe protein engineering of AEH via focused libraries and via machine-learning based techniques, such as FireProt and PROSS, to improve its thermal stability. By mutating up to 30% of AEH residues, we succeeded in dramatically stabilizing the enzyme but at the expense of activity. Only the (re) discovery of a Ca2+-binding site in X. campestris AEH reliably recovered activity.
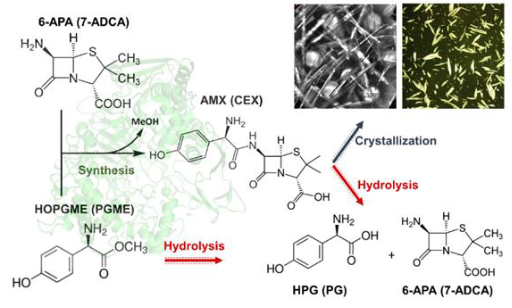
Figure: Enzymatically catalyzed synthesis and crystallization of amoxicillin, and primary and hydrolysis side reactions
For scale-up, accurate enzyme kinetics are crucial. Reactor performance in beta-lactam antibiotics synthesis is strongly impacted by minor enzyme inhibition that had not been detected under laboratory conditions. Data analysis employing Bayesian analysis and neural ODEs enable the formulation of a more robust kinetic model with given experimental data. Again, we will present newest results and insights.
About the Speaker(s)
 Andreas (Andy) S. Bommarius is a professor of Chemical and Biomolecular Engineering at the Georgia Institute of Technology in Atlanta, GA. He received his diploma in Chemistry at the Technical University of Munich, Germany (1984) and his Chemical Engineering B.S. (1982) and Ph.D. (1989) degrees at MIT, Cambridge, MA. At Georgia Tech since 2000, after 10 years as head of enzyme technology at Degussa/Evonik, his research interests cover biomolecular engineering, specifically biocatalyst development and protein/amyloid stability studies, continuous manufacturing, and Green Chemistry. His lab applies data-driven protein engineering to improve protein properties. Molecular foci are redox biocatalysts and beta-lactam amidases, process foci are multiphase reactors (gas/liquid and solid/liquid) and process stability studies (enzyme deactivation kinetics). Andreas (Andy) S. Bommarius is a Fellow of AIChE (2022) and AIMBE (2008). As of 2023, he heads the Georgia Tech ‘Center for a Renewables-based Economy from Wood’ (ReWOOD).
Andreas (Andy) S. Bommarius is a professor of Chemical and Biomolecular Engineering at the Georgia Institute of Technology in Atlanta, GA. He received his diploma in Chemistry at the Technical University of Munich, Germany (1984) and his Chemical Engineering B.S. (1982) and Ph.D. (1989) degrees at MIT, Cambridge, MA. At Georgia Tech since 2000, after 10 years as head of enzyme technology at Degussa/Evonik, his research interests cover biomolecular engineering, specifically biocatalyst development and protein/amyloid stability studies, continuous manufacturing, and Green Chemistry. His lab applies data-driven protein engineering to improve protein properties. Molecular foci are redox biocatalysts and beta-lactam amidases, process foci are multiphase reactors (gas/liquid and solid/liquid) and process stability studies (enzyme deactivation kinetics). Andreas (Andy) S. Bommarius is a Fellow of AIChE (2022) and AIMBE (2008). As of 2023, he heads the Georgia Tech ‘Center for a Renewables-based Economy from Wood’ (ReWOOD).
